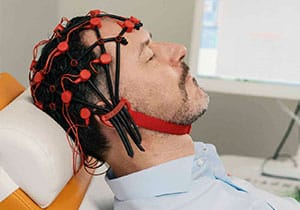
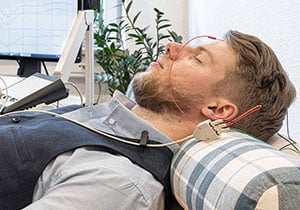
Vagus stimulation
In this procedure, gentle electrical impulses are applied to the left vagus nerve and sent to the brain via this nerve.
What is vagus stimulation?
Vagus nerve stimulation (VNS) is approved in Europe for the treatment of drug-resistant epilepsy (MRE) and refractory depression. The treatment is carried out by stimulating the vagus nerve. The vagus nerve is the tenth of a total of twelve cranial nerves and innervates several organs such as the heart, lungs and gastrointestinal tract. VNS was first approved in the European Union in 1994 and three years later in the USA. In 2010, a system for transcutaneous vagus nerve stimulation (t‑VNS) was approved in Europe.
Areas of application
Non-invasive vagus nerve stimulation (nVNS) is a novel non-drug and non-surgical treatment method for patients with
- drug-induced headache (so-called drug-overuse headache)
- Migraine with and without aura
- Cluster headache (Bing Horton headache)
- Hemicrania acutea
- Tension headache (episodic and chronic)
with the help of a patient-friendly, portable device that emits mild electrical signals.